
In the rule of chemical reactions, the changes in entropy occur as a result of the rearrangement of atoms and molecules that change the initial order of the system. A system with a great degree of disorderliness has more entropy.Įntropy is a factor of state function i.e., its value does not dependent on the pathway of the thermodynamic process and it acts as the determinant of only the initial and final state of the system. That is why we calculate the Entropy Change.Įntropy Change can be defined as the change in the state of disorder of a thermodynamic system that is associated with the conversion of heat or enthalpy into work.
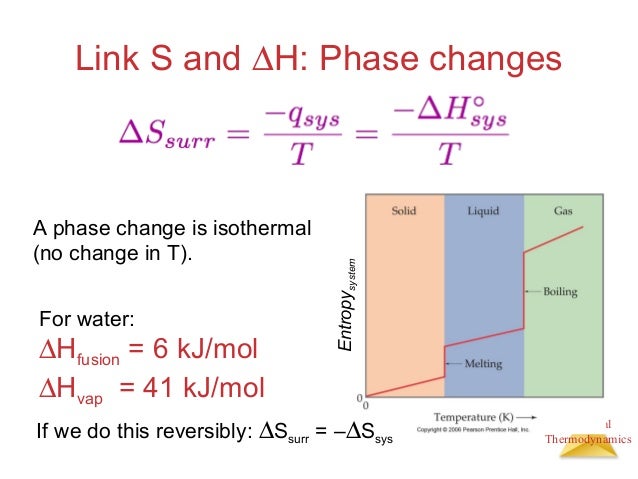
Entropy gives us a measure of that.Īs is clear from the law of thermodynamics that energy can neither be created nor destroyed but can be converted from one form to another, it is not possible to signify entropy at a single point, and hence, it can be measured as a change. Since energy gives the ability to get work done, it is practically impossible for all the energy to be used in doing work. In simpler words, entropy gives us an idea about that portion of energy that does not convert into work done and adds to the disorder of the system instead. the more is the spontaneity in a thermodynamic process, the higher is its entropy or the degree of disorder. It helps redefine the second law of thermodynamics.Įntropy relates to spontaneity i.e. Entropy is an interesting concept as it challenges the belief of complete heat transfer. The entropy is denoted by ‘S’ and it is an extensive property because the value of entropy or Entropy Change is dependent on the substance present in a thermodynamic system. The term disorder denotes the irregularity or lack of uniformity of a thermodynamic system. This randomness could be in regards to the entire universe or a simple chemical reaction or something as simple as the heat exchange and heat transfer.

So, let's understand this concept of entropy and the change in entropy.Įntropy is the measure of disorder or randomness. By this law, the entropy of the universe can never be negative. The second law of thermodynamics talks about the concept of entropy and tells that the entropy of the universe is always increasing. Three laws govern the science of thermodynamics and here we will discuss the second law of thermodynamics.
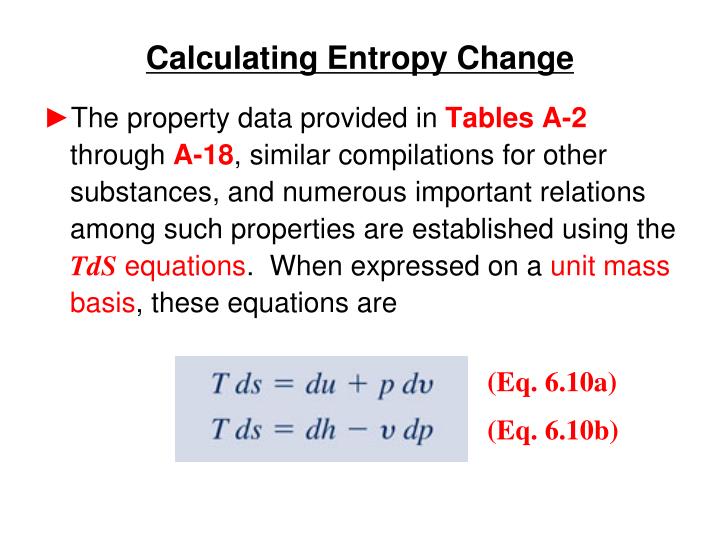
It also deals with the work done for the conversion of energy from one form to another. Thermodynamics is the study of the changes in energy associated with the change in temperature and heat.


 0 kommentar(er)
0 kommentar(er)
